Background: Foundational studies have revealed essential oncogenic pathways in DLBCL, triggering the development of drugs targeting distinct survival pathways in this malignancy. While many of these agents are active in DLBCL as monotherapy, they rarely induce deep responses or cure. Based on our identification of drug synergy in DLBCL models, we hypothesized that targeting multiple survival pathways concurrently could be curative in DLBCL. We developed a 5-drug combination regimen (ViPOR) that targets DLBCL survival sustained by constitutive B-cell receptor (BCR) signaling (ibrutinib, lenalidomide, prednisone) and by BCL2 (venetoclax), and also enlists the innate immune system using obinutuzumab. To maximize drug exposure and minimize toxicity, we administered all agents in non-continuous cycles for fixed duration in R/R DLBCL.
Methods: R/R DLBCL pts with adequate organ function were eligible. In Ph I, pts were treated at 4 doses of venetoclax (200-800 mg) PO D2-14 to identify the MTD. An initial 12d venetoclax ramp-up was given in combination with fixed-dose ibrutinib 560 mg PO D1-14, prednisone 100 mg PO D1-7, obinutuzumab 1000 mg IV D1-2, and lenalidomide 15 mg PO D1-14. Ph II expansion cohorts of R/R GCB and non-GCB DLBCL were included at the MTD. Max 6C of ViPOR q21d were given without maintenance. TLS, G-CSF, and PCP prophylaxis were given to all pts. Baseline CT, PET, BM, and tumor biopsies were performed with CT after C1, 2, 4, and 6 and PET after C6. CT was then performed q3m x 1y, q4m x 1y, q6m x 1y, then q12m x 2y. Tumor genomics and ctDNA (clonoSEQ) were studied.
Results: 50 DLBCL pts were enrolled (25 DLBCL NOS, 17 HGBCL-DH-BCL2, 3 HGBCL-DH-BCL6, and 5 THRLBCL). 52% and 48% were GCB and non-GCB subtype by IHC, respectively, with transformed lymphoma in 34%. Median age was 61y (range 29-77), with stage 3-4 disease in 92%, elevated LDH in 86%, >2 extranodal sites in 56%, and IPI >3 in 68% of pts. Median prior txs were 3 (range 1-9), with 40% post-CAR-T pts and 58% refractory.
A single DLT of G3 intracranial hemorrhage occurred, and venetoclax 800 mg was identified as the MTD. Heme AEs were most common, with G3-4 neutropenia in 24%, thrombocytopenia in 23%, and anemia in 7% of cycles. Febrile neutropenia occurred in 3 (1%) cycles. The only G3-4 non-heme AE in >10% pts was hypokalemia (28%). G3 A.fib occurred in 3 pts, and G4 TLS occurred in 1 pt, which resolved. Other common any grade non-heme AEs (% pts) included diarrhea (68%), hypokalemia (67%), nausea (45%), rash (35%), and fatigue (33%). Dose reductions occurred in 17% of pts, and 8% discontinued tx due to AE.
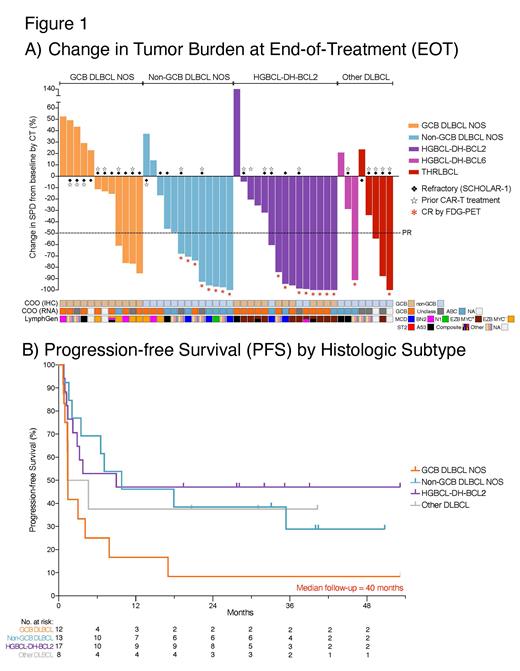
Of 48 evaluable pts (2 came off tx prior to restaging), ORR was 54% (26/48), and CR was 38% (18/48). Responses were observed across all molecular DLBCL subtypes, including a CR rate of 62% (8/13) in non-GCB DLBCL, 53% (8/15) in HGBCL-DH-BCL2, 25% (2/8) in other DLBCL, and a PR rate of 33% (4/12) in GCB DLBCL (non-DH) (Fig. 1A). CR rate was 20% (4/20) and 19% (5/27) in post-CAR-T and refractory pts, respectively.
With a median FU of 40m, 72% of CRs are ongoing, with a 2-year PFS and OS of 34% and 36%, respectively. By histology, 2-year PFS was 47%, 38%, 38%, and 8% in HGBCL-DH-BCL2, non-GCB DLBCL, other DLBCL, and GCB DLBCL (non-DH), respectively (Fig. 1B). 2-year PFS was 30% and 21% in post-CAR-T and refractory pts, respectively.
MRD was undetectable in 38% (16/42) of pts at end of therapy (EoT), and in 93% (14/15) of pts in PET CR at EoT. Elevated baseline ctDNA or detectable ctDNA during or at EoT were associated with significantly inferior PFS and OS, as were quantitative PET parameters (elevated baseline TMTV and TLG).
Two genetic subtypes known to rely on BCR-dependent NF-kB signaling - MCD and N1 - had a significantly higher CR rate (5/6, 83%) than all other genetic subtypes (4/22, 18%; p=0.0066).
Conclusions: This is the first study to show the feasibility and curative potential of multi-targeted therapy in R/R DLBCL. ViPOR was well tolerated across all ages in R/R DLBCL with rare febrile neutropenia. ViPOR was most effective in non-GCB DLBCL, as expected from its reliance on BCR signaling and BCL2. ViPOR was also highly active in HGBCL-DH-BCL2, possibly due to inhibition of MYC-driven apoptosis by BCL2 in this subtype. Durable remissions and likely cure were observed in non-GCB DLBCL (38%) and HGBCL-DH-BCL2 (47%), including pts relapsed after or refractory to CAR-T (30%). Multicenter Ph II testing is in development to confirm the activity of ViPOR in R/R non-GCB DLBCL and HGBCL-DH-BCL2.
OffLabel Disclosure:
Portell:Acerta/AstraZeneca: Research Funding; AbbVie: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; Jansen: Honoraria; Merck: Honoraria, Research Funding; SeaGen: Research Funding; Loxo/Lilly: Research Funding. Jacob:Adaptive Biotechnologies: Current Employment, Current equity holder in publicly-traded company. Simmons:Adaptive Biotechnologies: Current Employment, Current equity holder in publicly-traded company.
ViPOR is not FDA-approved for R/R DLBCL.